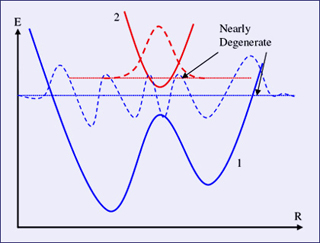
The Born-Oppenheimer approximation fails when two electron states almost cross, also known as an "avoided crossing." An avoided crossing is illustrated in the above plot of adiabatic electronic energy as a function of R. (Figure by Prof. Troy Van Voorhis.)
Instructor(s)
Prof. Troy Van Voorhis
MIT Course Number
5.73
As Taught In
Fall 2005
Level
Graduate
Course Description
Course Features
Course Description
5.73 covers fundamental concepts of quantum mechanics: wave properties, uncertainty principles, Schrödinger equation, and operator and matrix methods. Basic applications of the following are discussed: one-dimensional potentials (harmonic oscillator), three-dimensional centrosymmetric potentials (hydrogen atom), and angular momentum and spin. The course also examines approximation methods: variational principle and perturbation theory.
Other Versions
Other OCW Versions
OCW has published multiple versions of this subject. ![]()


