2.1 - Competent Chemist Rating
"Ethyl Ester's Excellent Adventure"
Techniques Checklist
- Extraction
- Melting point determination
- Solvent drying and concentration
- Nuclear Magnetic Resonance (NMR) spectrometer operation
- Infrared (IR) spectrometer operation
- Careful transfer of solutions without loss of material
Pre-lab Discussion
- Extraction—Reading: Zubrick chapter 15, LLP chapter 10, Mohrig chapter 11
- Theory of extraction—Reading: Zubrick chapter 35
- Melting point determination—Reading: Zubrick pages 88–103, Mohrig 176–180
- NMR theory and operation—Reading: Zubrick chapter 33, LLP chapter 15.2, Mohrig chapter 21
- IR theory and operation—Reading: Zubrick chapter 32, LLP chapter 15.3, Mohrig chapter 20
Digital Lab Techniques Manual
- Video 5, Reaction Work-Up I (Extracting, Washing and Drying)
- Video 6, Reaction Work-Up II (Using the Rotovap)
- Video 11, Using a balance
- Video 12, Melting Point Determination
Equipment
- Separatory funnel—250 or 500-mL
- Graduated Cylinder—100-mL
- Erlenmeyer flasks—2x250-mL, 1x500-mL
- Beaker 150-mL
- Round-bottomed flasksv50-mL, 100-mL
- NMR tubes
- IR cards
- Funnels—large, with long neck
- Filter Paper
- Rotary evaporator
Goal
To manipulate and purify a known amount of a contaminated sample and to record its 1H NMR and IR spectra, all with minimal loss of material.
Experiment Outline
- You will receive a vial containing 100 mg of ethyl 3-hydroxybenzoate (mp 72–74 °C) contaminated with triethylamine. You will also receive four different 1H NMR spectra of: the mixture in your vial, pure ethyl 3-hydroxybenzoate, pure triethylamine, and diethyl ether.
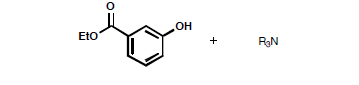
- Dissolve the sample in 50–75 mL of ether in a separatory funnel.
- Remove the amine by extraction with a 10% HCl solution.
- Continue with the "standard aqueous work-up" (including an ether back extraction)—see Extraction and Washing Guide.
- Remove the solvent by rotary evaporation and concentration under vacuum to a constant weight and obtain a mass.
- Take an NMR spectrum of the compound and compare to your earlier spectra.
- Recombine the NMR sample with the remainder of the purified sample.
- Obtain an IR spectrum using your IR card—see IR Sample Prep. Guide.
- Remove the solvent for the final time to a constant weight.
- Obtain a mass and a melting point.
Notes
- When removing solvent with the rotary evaporator, keep the receiving flask cold and the water bath warm. Otherwise, your product will never solidify.
- If you have trouble getting your product to solidify, add a few milliliters of methylene chloride to your flask and return it to the rotary evaporator.
Results
- To obtain your "CC Rating" in Transfer and Extraction Techniques, you must end with at least 90 mg of ethyl 3-hydroxybenzoate. Additionally, this material must be of adequate purity as determined by IR and 1H NMR analysis (spectra should show only negligible amounts of impurities in the judgment of the professor and TA) and by melting point measurement (should melt over no more than three degrees with the lower range beginning no lower than 69 °C and the upper range ending no higher than 73 °C). This material must also be submitted to the TA for possible weight and melting point confirmation measurements.
