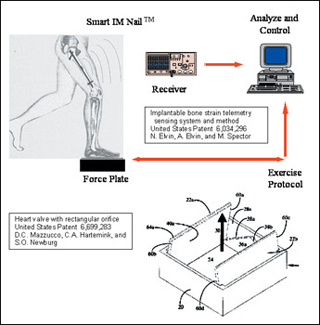
Two of the patents resulting from projects of 2.782J design teams in prior years: implantable bone strain telemetry sensing (Elvin et al, 1997) and a heart valve with rectangular orifice (Mazzucco et al, 2000). (Image by Prof. Myron Spector.)
Instructor(s)
Prof. Ioannis Yannas
Prof. Myron Spector
MIT Course Number
2.782J / 3.961J / 20.451J / HST.524J
As Taught In
Spring 2006
Level
Graduate
Course Description
Course Features
Course Description
This design course targets the solution of clinical problems by use of implants and other medical devices. Topics include the systematic use of cell-matrix control volumes; the role of stress analysis in the design process; anatomic fit, shape and size of implants; selection of biomaterials; instrumentation for surgical implantation procedures; preclinical testing for safety and efficacy, including risk/benefit ratio assessment evaluation of clinical performance and design of clinical trials. Student project materials are drawn from orthopedic devices, soft tissue implants, artificial organs, and dental implants.


