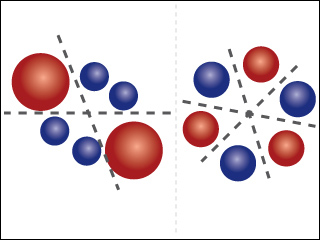
Molecular orbitals of benzene, with positive lobes shown in blue, and negative lobes shown in red. Learn more about molecular orbitals in Lecture 27. (Original image by Professor Troy Van Voorhis, adapted by MIT OpenCourseWare.)
Instructor(s)
Prof. Troy Van Voorhis
Prof. Robert Field
MIT Course Number
5.61
As Taught In
Fall 2013
Level
Undergraduate
Course Description
Course Features
Course Description
This course presents an introduction to quantum mechanics. It begins with an examination of the historical development of quantum theory, properties of particles and waves, wave mechanics and applications to simple systems—the particle in a box, the harmonic oscillator, the rigid rotor and the hydrogen atom. The lectures continue with a discussion of atomic structure and the Periodic Table. The final lectures cover applications to chemical bonding including valence bond and molecular orbital theory, molecular structure, and spectroscopy.
Acknowledgements
The material for 5.61 has evolved over a period of many years, and, accordingly, several faculty members have contributed to the development of the course contents. The original version of the lecture notes that are available on OCW was prepared in the early 1990's by Prof. Sylvia T. Ceyer. These were revised and transcribed to electronic form primarily by Prof. Keith A. Nelson. The current version includes additional contributions by Professors Moungi G. Bawendi, Robert G. Griffin, Robert J. Silbey, and John S. Waugh, all of whom have taught the course in the recent past.
Other Versions
Other OCW Versions
OCW has published multiple versions of this subject. ![]()


